The December 15, 2025 Health and Human Services (HHS) “Invisible Illness” Lyme Disease Roundtable marked a pivotal federal acknowledgment that Lyme disease is a serious, chronic, biologically based illness and that the era of invalidating patients must end. As Director of the Johns Hopkins Lyme Disease Research Center and Clinical Care Program, I strongly affirm these conclusions and believe our program offers a proven multidisciplinary clinical research model for nationwide Clinical Centers of Excellence that can help guide and accelerate national progress in diagnosis, treatment, and improved long-term outcomes.
Key messages from the HHS roundtable
The roundtable centered the voices of Lyme disease patients and front-line clinicians, underscoring that meaningful progress must begin with validating lived experience and listening carefully to those living with persistent symptoms and the healthcare providers caring for them. Federal leaders, including Robert F. Kennedy Jr, Secretary of the US Department of Health and Human Services, and Jay Bhattacharya, MD, PhD, Director of the National Institutes of Health, highlighted renewed commitments to public-private partnerships, such as the Steven & Alexander Cohen Foundation’s LymeX diagnostic testing program, and major policy shifts such as the development of Clinical Centers of Excellence, signaling an end to decades of neglect, diagnostic uncertainty, and treatment inadequacy. Speakers emphasized that existing diagnostics and short-course treatments fall short for many patients, and called for data-driven innovation, including direct-detection tests, multi-omic approaches, AI tools, and rigorously designed clinical trials.
How the Johns Hopkins multidisciplinary model aligns with HHS priorities
For a decade, the Johns Hopkins Lyme Disease Research Center has been organized around the same principles highlighted by the roundtable: compassionate, continuity-based care for all stages of Lyme disease and a tight integration of patient care with rigorous translational research. The Center’s multidisciplinary clinical research program brings together infectious diseases, rheumatology, neuropsychiatry, neurology, neuropsychology, and autonomic specialists to care for patients with acute infection, persistent symptoms of pain, fatigue, dysautonomia, neurocognitive changes, and neuropsychiatric manifestations. Thousands of patients have received individualized, science-driven care through this model, which functions as a living laboratory to inform better diagnostics and treatments.
Bridging care, cohorts, and discovery
The Center’s SLICE studies and biorepository provide one of the most extensive, longitudinal, well-characterized cohorts of patients with acute and chronic Lyme disease, with more than 895 participants and over 117,000 carefully curated blood and tissue samples. This “gold standard” resource has enabled discovery of immune dysfunction, biomarkers of persistent symptoms, neuroimaging signatures of brain involvement, and identifiable risk for autoimmune inflammatory arthritis and dysautonomia, directly addressing the scientific priorities raised at the roundtable. Through 84 peer-reviewed publications and 45 external research collaborations across 31 institutions, the Center has helped shift clinical and scientific consensus toward recognizing Lyme disease associated chronic illness as a real, complex, biological condition rather than a psychosomatic disorder. The Center’s research has also shown that contested narratives and illness invalidation by medical professionals is a common problem for Lyme patients.
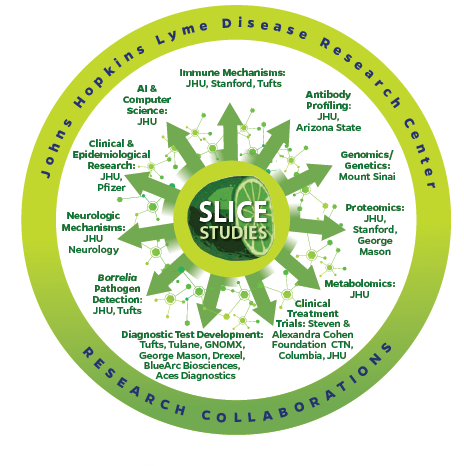
Advancing diagnostics, trials, and multidisciplinary care
The roundtable’s call for better diagnostics and robust clinical trials is directly aligned with the Center’s ongoing work to develop new tests and evaluate targeted treatments. Our Center’s investigators collaborate widely on next-generation diagnostics, including pathogen detection, immunologic profiling, metabolomics, and AI-enabled rash recognition, and have helped launch treatment trials, such as an extended tetracycline pilot for post-treatment Lyme disease through the Steven & Alexandra Cohen Foundation’s Clinical Trials Network. The Center also leads programs in Lyme-associated dysautonomia, POTS, neuroimaging, and autoimmune arthritis, embodying the broader concept that Lyme and related infection-associated chronic conditions and illnesses (IACCI) require multidisciplinary frameworks that integrate immunology, neurology, psychiatry, infectious disease and rheumatology to address overlapping mechanisms that drive disease.

Clinical Trials Network as a model for Clinical Centers of Excellence
I support the creation of a federally funded national network of Lyme Disease Clinical Centers of Excellence to coordinate and expand innovation, clinical care, and education. In this vision, clinical research centers such as ours at the Johns Hopkins Lyme Disease Research Center, together with our collaborators in the Clinical Trials Network, including the Lyme and Tick-borne Diseases Clinical Trials Coordinating Center at Columbia University, Children’s National Medical Center, UCSF, SUNY Upstate Medical University, The Cohen Center for Recovery from Complex Chronic Illness at the Icahn School of Medicine at Mount Sinai, Hackensack-Meridian Health in New Jersey, and the University of North Carolina at Chapel Hill, could help build a foundation for sustainable, federally supported multicenter clinical trials. These centers would also provide standardized and accessible front-line care, maintain well-characterized patient cohorts and biorepositories, and train the next generation of clinician-scientists in infection-associated chronic illnesses. In addition, real-world data on symptoms and treatment outcomes from diverse clinical practices that care for Lyme patients should also be collected and analyzed to help inform priorities for multicenter clinical trials.
By validating patients, rejecting outdated psychosomatic framing, and investing in multidisciplinary IACCI-oriented Clinical Centers of Excellence and large-scale clinical trials, the nation can ensure that the momentum of the HHS roundtable becomes measurable progress in diagnostics, treatments, and recovery for the millions affected by Lyme disease and related conditions.


